TCX-101 Program
BlueSphere’s TCX-101 program combines an engineered TCR T-cell therapy targeting a blood restricted antigen with allogeneic stem cell transplant. Our goal is to create a best-in-class HLA population coverage panel of novel TCR T-cell therapies for treatment of AML, ALL, and MDS.
Our first clinical candidate, BSB-1001, targets the blood restricted antigen HA-1
Discovery
IND-ENABLING
PHASE 1
HEMATOLOGIC
Discovery
IND-Enabling
PHASE 1
BSB-1001: Blood Restricted #1 – HA-1
BSB-1001: Blood Restricted #1 – HA-1
Enrolling
Discovery
IND-Enabling
PHASE 1
Blood Restricted #2
Blood Restricted #2
Discovery
IND-Enabling
PHASE 1
Blood Restricted #3
Blood Restricted #3
Discovery
IND-Enabling
PHASE 1
Blood Restricted #4
Blood Restricted #4
The TCX-101 program used our TCXpressTM platform to identify novel TCRs targeting several different blood restricted antigens. These TCRs are then engineered into T cells.
How does a TCR T-cell therapy work?
- Unlike CAR-T cells, TCR T-cell therapy can target intracellular antigens.
- T cells kill targeted cells by using their T cell receptor to identify fragments of antigens (peptides) presented by Human Leukocyte Antigens (HLA) molecules expressed by target cells. Think of this as a ‘lock and key’ system.
- The TCR on the T cells acts as the key and the HLA-peptide on the target cell acts as the lock. If a T cell ‘key’ finds the right ‘lock’, it destroys the cell.
- T cells can be engineered to have specific keys and given to patients as TCR T-cell therapy. When given to patients that have cancerous cells with a corresponding lock, then the TCR T-cell therapy works to kill those cancerous cells.
How does the blood-restricted antigen, HA-1, play a role in fighting cancer?
- HA-1 is an antigen presented only by a patient’s blood cells, including leukemia cells.
- BSB-1001 are T cells engineered to kill any cell presenting the HA-1 antigen.
- Evidence exists showing that HA-1 (and other ‘like’ antigens) directed T cells are responsible for anti-leukemia effect (Marijt, W., et al. PNAS 2003)
- BSB-1001 expected NOT to target other tissues or cell types, minimizing the risk of off-tumor effects
- BlueSphere has developed best-in-class population coverage by identifying TCRs targeting four different blood-restricted antigens
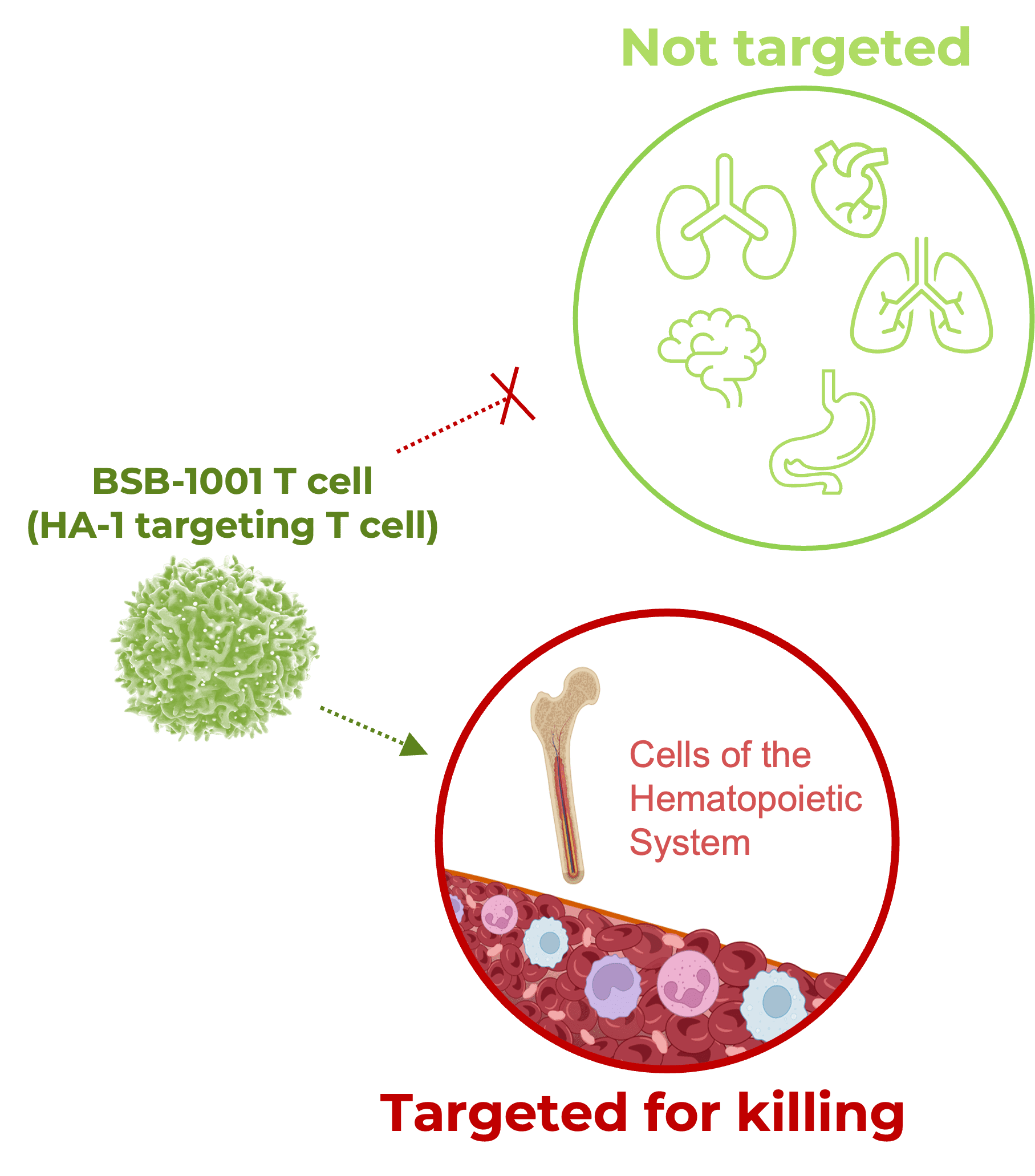
What about the patient’s non-cancerous blood cells getting destroyed by this TCR T-cell therapy?
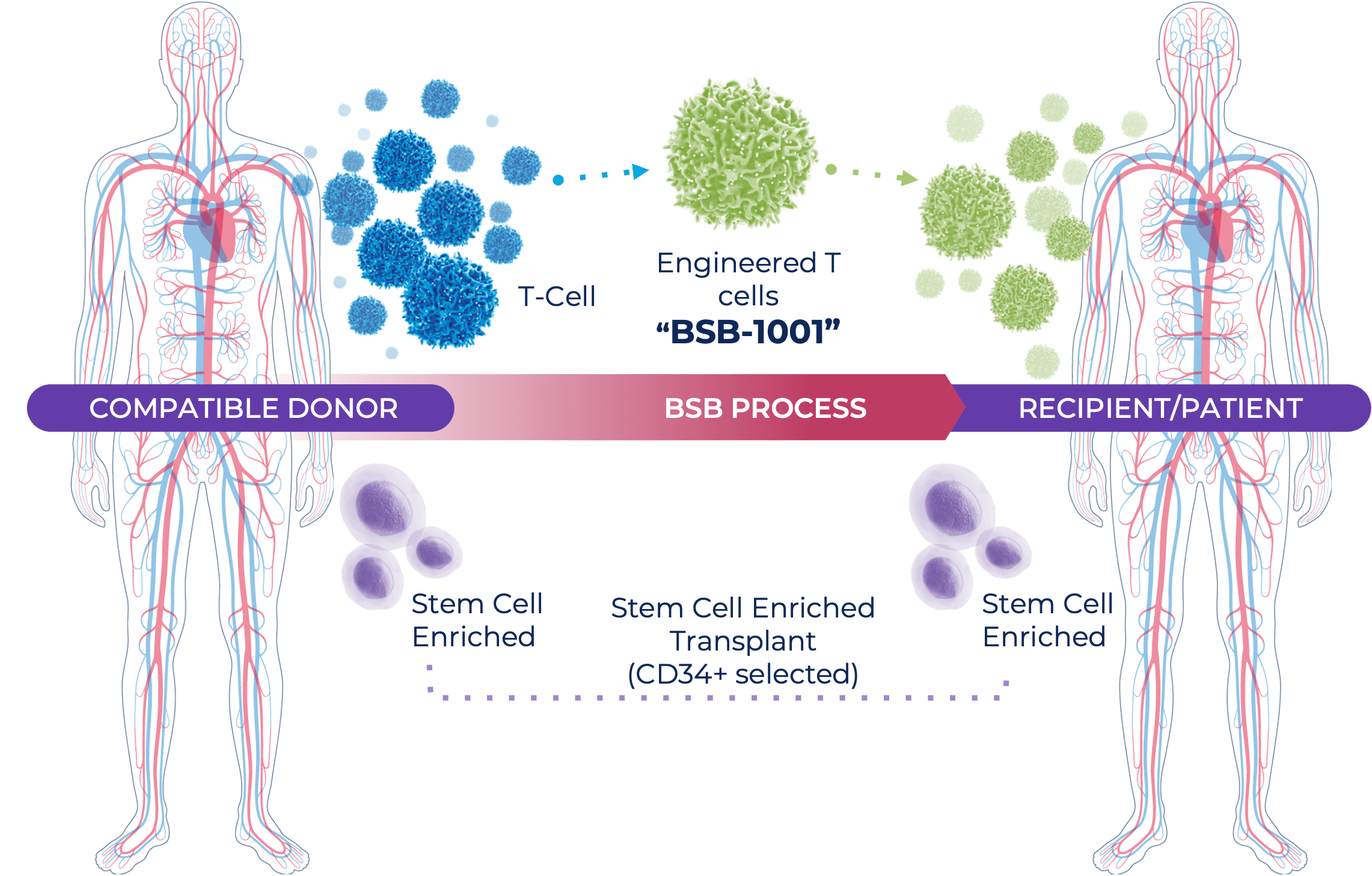
- In order to provide the patient with replacement blood stem cells, the TCX-101 therapy is paired with a CD34+ (only stem cells) allogeneic stem cell transplant.
- The stem cell transplant comes from a donor who does NOT present HA-1 on their blood cells, which allows the transplanted cells to escape being destroyed by the BSB-1001 therapy.
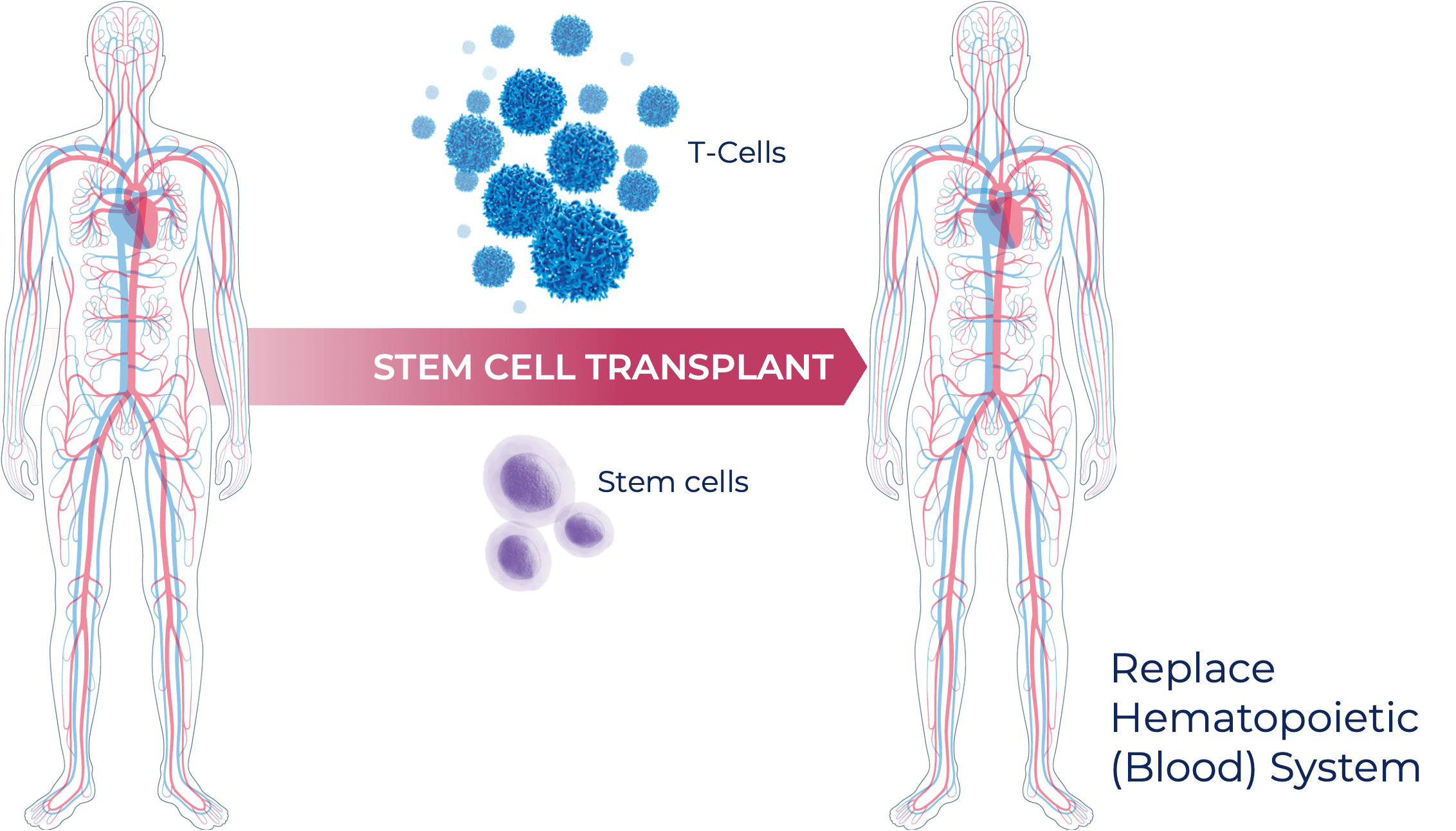
What is an allogeneic stem cell transplant?
- Allogeneic stem cell transplant (alloSCT) is the standard of care used to treat high-risk leukemias.
- These transplants typically come from a mixture of donor cells, including both stem cells and T cells that are used to replace the patient’s entire blood system.

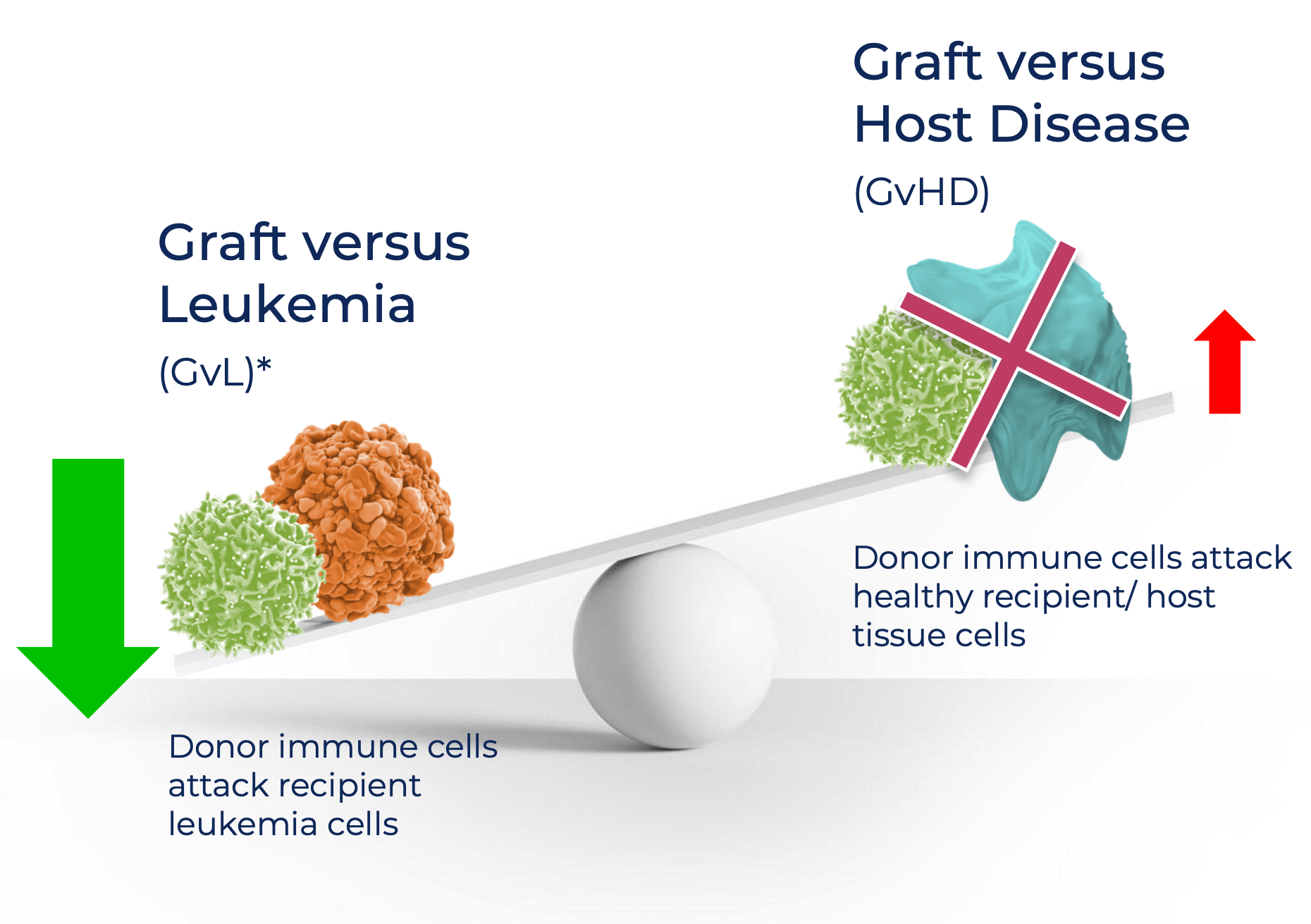
- Unfortunately, while some of the T cells in the transplant can attack cancerous cells, leading to graft versus leukemia (GvL), the majority of the T cells give rise to graft versus host disease (GvHD), which is a serious side effect of stem cells transplant. This is where the donor T cells attack the patient’s healthy tissues.
- However, some T cells are known to kill leukemia (graft v leukemia), which is the concept behind BSB-1001.
- BlueSphere’s BSB-1001 therapy is comprised of modified T cells that can kill the patient’s blood cells, including any remaining leukemia cells, but that do not target other healthy cells, thereby enhancing GvL and minimizing GvHD.
- The patients still receive the neutral stem cells (CD34+ alloSCT) to repopulate a healthy, cancer-free hematopoietic system.
Why does BlueSphere believes this approach will be unique?
- BSB will treat patients who are currently eligible for transplant AND patients with ACTIVE leukemia, who are not typically eligible for transplant due to their high risk of recurrence.
- BSB will treat patients on Day 0, at the same time as alloSCT. By doing this, we should achieve better T cell activation and less chance of residual leukemia presence, ultimately leading to better efficacy.
- Because BSB deletes the native TCR (increased safety) and do a CD34 transplant (only donor stem cells without non-engineered T cells), BSB will not use immunosuppression. This allows for higher chance of killing leukemia and less chance of GVHD.
Contact us to hear more about our clinical programs and technology.
